What Does Spin Quantum Number Determine
A spin magnetic quantum number determines the spin of an electron. It is denoted by s.
 What Is The Spin Quantum Number Quora
What Is The Spin Quantum Number Quora
The only possible values of a spin quantum number are or - sometimes referred to as spin up and spin down.

What does spin quantum number determine. The Spin Quantum Number ms describes the angular momentum of an electron. Denoted as m s the electron spin is constituted by either upward m s 1 2 or downward m s 1 2 arrows. In practice spin is given as a dimensionless spin quantum number by dividing the spin angular momentum by the reduced Planck constant which has the same dimensions as angular momentum although this is not the full computation of this value.
Electron Spin or Spin Quantum Number is the fourth quantum number for electrons in atoms and molecules. Describes the electron spin. 4 rows Quantum number is the number by which we can identify the position of a particular revolving.
The fourth quantum number describes the spin intrinsic angular momentum of the electron within that orbital and gives the projection of the spin angular momentum s along the specified axis. There are only two possible values. It describes the quantum state of an electron including its energy orbital shape and orbital orientation.
Deals with the orientation of the atomic orbital in 3D space. The spin quantum number is the quantum number that describes the orientation of the intrinsic angular momentum of an elementary particle. How do you predict the value of nuclear spin I based on the number of protons and neutrons.
The spin quantum number describes the spin for a given electron. Across the entire periodic table nuclear spin values ranging from I 0 to I 8 in -unit increments can be found. The only real significance to the spin quantum numbers is to indicate that any two electrons in an orbital must have opposite spin.
Values are - 0. Spin quantum number m s. M magnetic quantum number the orbital in the sublevel.
spin up electron or spin down electrons. The possible values for the principal quantum number that is n are 0 1 2 and 3. Spin is an intrinsic property of an elementary particle which is responsible for the spin angular momentum.
Very often the spin quantum number is simply called spin. The spin quantum number also known as the fourth quantum number is a number value that describes the orientation of an electron occupying an orbital. Because angular momentum is a vector the Spin Quantum Number s has both a magnitude 12 and direction or -.
We also represent spin with arrows or. It splits the sub-shells such as spdf into individual orbitals and places the electron in one of them. The second electron assigned to an orbital would be - 12.
Spin quantum number The magnetic quantum number is the third on the list between spin and azimuthal quantum number. Each orbital can only hold two electrons. By convention the first electron assigned to an orbital is assign a spin quantum number s 12.
An electron can have one of two associated spins 1 2 1 2 spin or 1 2 1 2 spin. When the spin quantum number of a nucleus is non-zero it possesses a magnetic moment. While the magnetic number determines how an atom may shift its movement when under the influence of an external energy source the spin magnetic quantum number reflects the strength of the atoms energy.
Similarly for subshell like p the value 1 is used. Ms spin quantum number electron in orbital values are or -. Analogously the values of m s range from s to s where s is the spin quantum number an intrinsic property of particles.
It defines the orientation in space of a given orbital of particular energy n and shape I. It has a spin I of 12 and when placed in a magnetic field of strength B0 it will occupy 2 I 1 quantized magnetic energy states in this case 2. The possible values for m l is the range of l.
Magnetic Quantum Number m l. An electron spins around an axis and has both angular momentum and orbital angular momentum. Protons and neutrons each have net spins of but this derives from the elementary quarks and gluons of which they are composed.
Generally for subshell s the value 0 is used. An electron cannot have zero spin. The spin quantum number indicates the orientation of the intrinsic angular momentum of an electron in an atom.
The proton is such a nucleus. The electron configuration gives the distribution of electrons within an element while the quantum number gives the probable location of those electrons.
Quantum Numbers Of The Wave Function Quantum Numbers
 How To Find The Quantum Numbers Of An Element Study Chemistry With Us Youtube
How To Find The Quantum Numbers Of An Element Study Chemistry With Us Youtube
 Magnetic Quantum Number Chemistrygod
Magnetic Quantum Number Chemistrygod
 Magnetic Quantum Number Chemistrygod
Magnetic Quantum Number Chemistrygod
 The Spin Quantum Number Flux Aufbau Principle Linear Momentum
The Spin Quantum Number Flux Aufbau Principle Linear Momentum
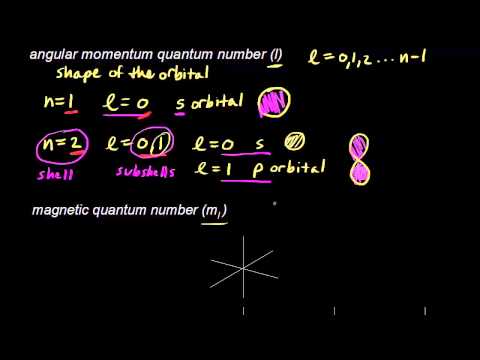 Quantum Numbers Video Quantum Physics Khan Academy
Quantum Numbers Video Quantum Physics Khan Academy
 Quantum Number An Overview Sciencedirect Topics
Quantum Number An Overview Sciencedirect Topics
Quatumnumbers Nuclear Chemistry
 The Spin Quantum Number Youtube
The Spin Quantum Number Youtube
 The Science Week Quantum Numbers Simplified Notes
The Science Week Quantum Numbers Simplified Notes
 Quantum Numbers Schrodinger S Equation Requires 3 Quantum Numbers Although There Are A Total Of 4 Quantum Numbers Ppt Video Online Download
Quantum Numbers Schrodinger S Equation Requires 3 Quantum Numbers Although There Are A Total Of 4 Quantum Numbers Ppt Video Online Download
Chemistry Life The Universe And Everything
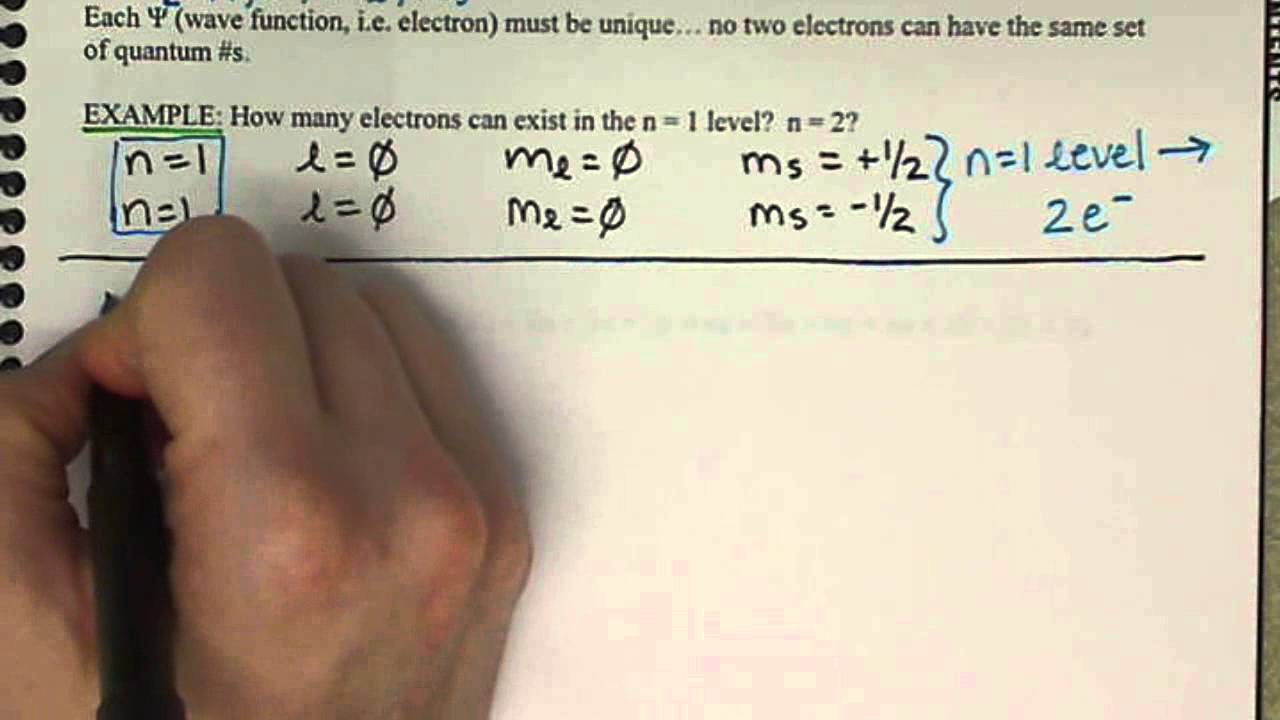 Quantum Numbers Spin Quantum Number Ms Chem161 7 6 Youtube
Quantum Numbers Spin Quantum Number Ms Chem161 7 6 Youtube
 Quantum Number Orbital Diagram Priyamstudycentre
Quantum Number Orbital Diagram Priyamstudycentre
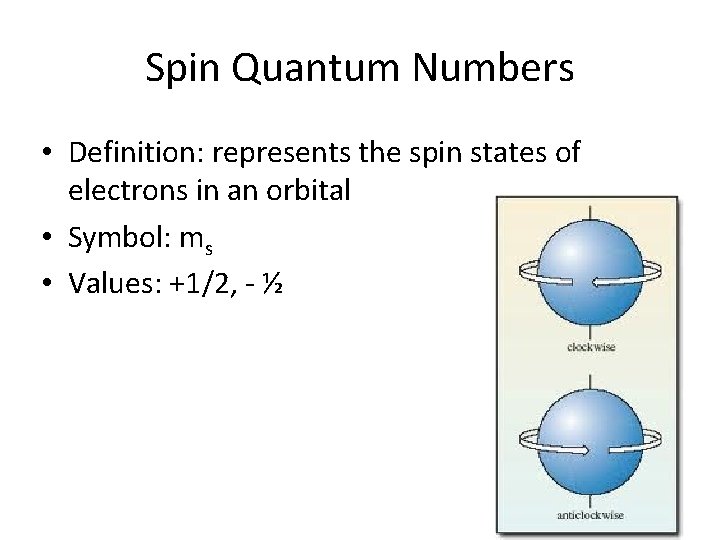 Quantum Numbers Activity Quantum Numbers Quantum Numbers Tell
Quantum Numbers Activity Quantum Numbers Quantum Numbers Tell
 Quantum Numbers And Rules Physics
Quantum Numbers And Rules Physics

Post a Comment for "What Does Spin Quantum Number Determine"